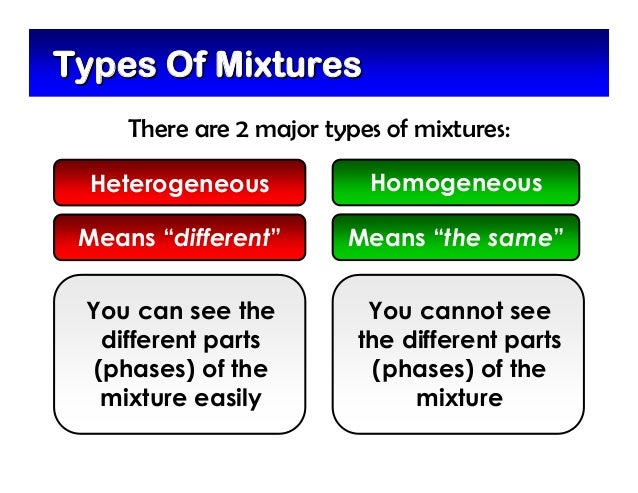
A homogeneous mixture has the same uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases The three phases or states of matter are gas, liquid, and solidThis video explains the difference between homogeneous and heterogeneous solutionsColloids are heterogeneous like suspensions but visually appear to be homogeneous because the particles in the mixture are very small—1 nanometer to 1 micrometer The difference between colloids and suspensions is that the particles in colloids are smaller and that the particles will not settle over time

The Density Bottle Strikes Again Chemical Education Xchange
What is difference between heterogeneous and homogeneous
What is difference between heterogeneous and homogeneous-"Heterogeneous solution" is a contradiction in terms, and "emulsion" is not Solutions are, by definition, homogeneous An emulsion is a colloidal dispersion of one liquid in another You can talk about a heterogeneous mixture, but not a heterogenWhile heterogeneous mixtures are uneven, with a composition that varies from one point to another In homogeneous mixtures, there seems to be only one component (solute and solvent), but in heterogeneous, we easily visualize more than two components




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous
Homogeneous mixtures are called solutions Once a solution is created, it cannot be separated by mechanical means Other examples of homogeneous mixtures Air, water and alcohol, water and sugar HETEROGENEOUS It refers to combinations that are not totally uniform and in many cases are clearly visible when "mixed"What are the difference between homogeneous and heterogeneous The terms à ¢ â ¬ Å Homogeneousà  »and à ¢ â ¬ Å Heterogeneousà ¢ â ¬ â ¢ words are commonly used in chemistry and refer to solutions and mixtures Any type of mixture can be divided as homogeneous or heterogeneousMany people enjoy a cup of coffee at some point during the day Some can drink black, while others can put cream (or some milk substitute) and sugar in their coffee
Examples of homogeneous mixture A glass of lemonade (mixture of water, lemon juice, sugar, salt) is a homogeneous mixture because the dissolved sugar, salt, and lemon juice are evenly distributed throughout the entire sample You can't easily separate the lemon juice from the water; Difference between Homogeneous and Heterogeneous Mixtures 1 Mixtures that have uniform composition Mixtures that do not have uniform composition throughout 2 Boundary of separation could not be seen Boundary of separation of constituent particles is clearly visible 3 Particles are not indistinguishable All solutions of the inhomogenous equation can be found by finding all solutions of the homogenous equation and then adding the particular solution $$ A (x_h x_p) = A x_h A x_p = 0 b = b $$ This is quite useful and is applied from linear differential equations to linear Diophantine equations
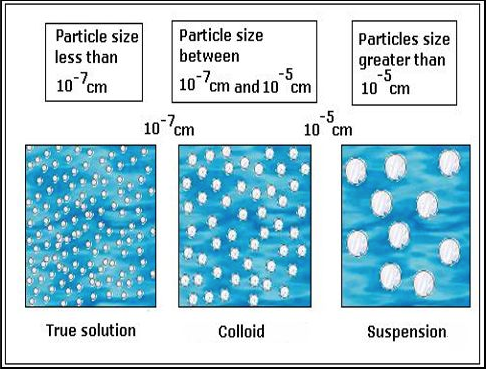
the terms heterogeneous and homogeneous are two different mixtures that refer to chemistry when two or more substances without a chemical reaction are mixed together, a mixture is formed the mixtures are very important in chemistry there are two major types of mixtures Homogeneous and heterogeneous mixtures the basic difference between heterogeneous and homogeneous Homogeneous mixtures are uniform, that is, their composition is the same wherever you look at it;Homogeneous Mixtures Heterogeneous Mixtures centrifugation coagulation distillation evaporation filtration hand picking magnetic separation sieving winnowing sedimentation Mixture Separation Techniques True solution Colloidal solutions Suspensions These solutions typically differ in the particle size of the solute SolutionBased Mixtures True




Explain The Difference Between A Homogeneous And Heterogeneous Mixture Give An Example For Each Brainly Com




Is Skim Milk A Homogeneous Mixture Essay Example
A homogeneous solution tends to be identical, no matter how you sample it Homogeneous mixtures are sources of water, saline solution, some alloys, and bitumen Sand, oil and water, and chicken noodle soup are examples of heterogeneous mixtures A homogeneous mixture has the same uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases Solutions have particles which are the size of atoms or molecules too small to be seenBasically we subdivide a mixture in homogeneous and heterogeneous mixtures In simple words, in a homogeneous mixture, it is not possible to easily differentiate between its components The components in a heterogeneous mixture can be easily differentiated An example of a homogeneous solution can be a salt solution



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms
When two or more substances (compounds or elements) mix together without showing any chemical change, they are called mixtures It generally has two major types based on the mixing of substances, such as homogeneous mixtures and heterogeneous mixturesBy the difference and similarity between the animals, a heterogeneous and homogeneous selection is distinguished Heterogeneous selection is applied when useful characteristics are not Heterogeneous selection is applied when useful characteristics are not A homogeneous mixture has the same uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases Solutions have particles which are the size of atoms or molecules too small to be seen




Module 4 Lesson 1




List The Points Of Difference Between Homogeneous And Heterogeneous Mixtures Brainly In
Homogeneous mixture Heterogeneous mixture 1) These are called as solutions These are called as suspensions/colloids 2) Substances are Uniformly distributed These substances are Unevenly distributed 3) These are not visible to the naked eye, but visible through the microscopeHomogeneous and heterogeneous are words to describe mixtures Mixtures are how the particles (compounds, atoms ions bulk components) are mixed togetherParticle size distinguishes homogeneous solutions from other heterogeneous mixtures Solutions have particles which are the size of atoms or molecules too small to be seen A colloid is a homogeneous solution with intermediate particle size between a solution and a suspension




Q2 Differentiate Between Homog Lido



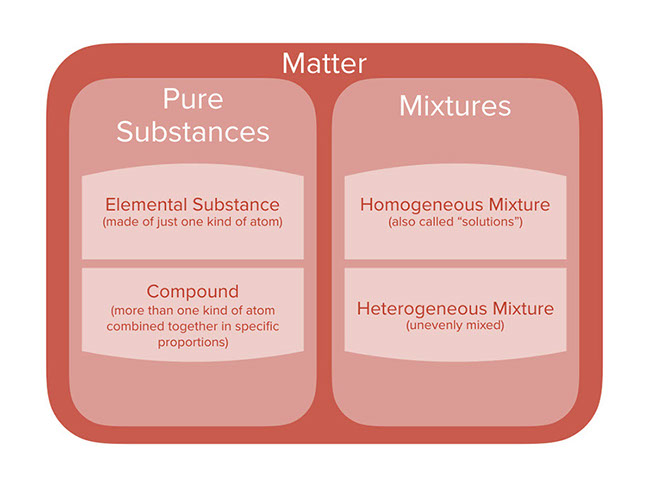
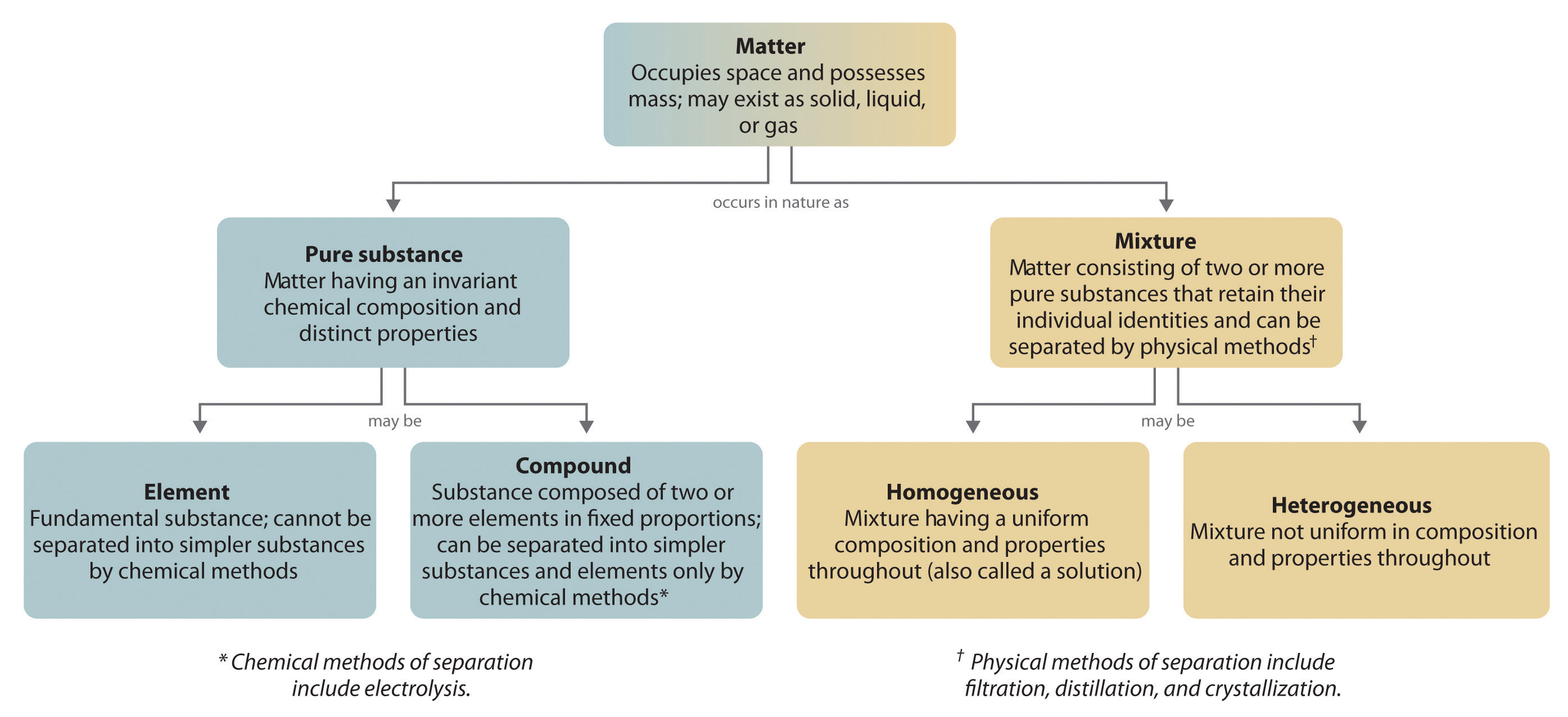
Mixture
Difference between compound and homogeneous mixture learning objectives explain the difference between a pure substance and a mixture explains the difference between an element and a compound explains the difference between a homogeneous mixture and a heterogeneous mixture a useful way to organize our understanding of matter is to think of a hierarchy thatDescription They are generally referred to as solutions They are generally referred to as suspensions/colloids Purity Homogenous mixtures are pure substances Heterogeneous substances are not pure substances Phase The whole mixture is in a single (same) phase Substances can consist of two phases and layers A homogeneous mixture is a mixture having a uniform composition throughout the mixture For example salt in water, sugar in water, copper sulphate in water A heterogeneous mixture is a mixture having a nonuniform composition throughout the mixture For example sodium chloride and iron fillings, salt and sulphur, oil and water



Sci8u1l2




Comparison Of Heterogeneous And Homogeneous Catalysis Download Table
Difference Between Heterogeneous and Homogeneous We come across homogeneous and heterogeneous products in our everyday lives Basically we subdivide a mixture into homogeneous and heterogeneous mixtures In simple words, in a homogeneous mixture, you cannot differentiate the its components easily Components in a heterogeneous mixture can be Examples of a heterogeneous mixture Colloid A colloid is an example of a heterogeneous mixture where the components exist in two distinct phases; The homogenous mixture has one phase, while the heterogenous has two or more phases A homogenous mixture is commonly referred to as a solution An example of a homogenous mixture is alcohol and water, while an example of a heterogeneous mixture



Lesson Categories Of Chemicals And Mixtures



Matter Pure Substance Element Compound Mixture Homogeneous Solution
Difference between Homogeneous and Heterogeneous Key Difference Homogenous refers to a solution that is a completely uniform mixture of two or more objects Heterogeneous refers to solutions that are not completely uniform and in most cases is clearly visible when viewing the mixture The key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do not have either uniform composition or uniform properties The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughout




What Are Mixtures Definition Overview Expii




What Is A Homogeneous Mixture Definition And Examples
Here to find the difference between homogeneous and heterogeneous mixtures? What is the difference between heterogeneous and homogeneous? A diagram representing at the microscopic level the differences between homogeneous mixtures, heterogeneous mixtures, compounds, and elements Solution edit A solution is a special type of homogeneous mixture where the ratio of solute to solvent remains the same throughout the solution and the particles are not visible with the naked eye
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures



What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com
A homogeneous mixture has the same uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases Solutions have particles which are the size of atoms or molecules – too small to be seenThis video is in simple language about Difference between homogenous and heterogeneous mixturesClass 9Chapter 2Is Matter Around Us PureA homogenous mixture is that mixture in which the components mix with each other and its composition is uniform throughout the solution A heterogenous mixture is that mixture in which the composition is not uniform throughout and different components are observed




Crossing The Divide Between Homogeneous And Heterogeneous Catalysis In Water Oxidation Pnas




Chemistry For Kids Chemical Mixtures
Problem What is the difference between a homogenous and a heterogeneous mixture?(a) A homogeneous mixture has the same composition throughout, while a heterogeneous mixture has different compositions in different regions(b) A homogeneous mixture consist of only one compound, while a heterogeneous mixture consist of two or more compounds(c) A homogeneous The terms homo and hetero indicate the most prominent differences between homogeneous and heterogeneous mixtures The prefix refers to homo while homo indicates homosexual nonhomogeneity Homogeneous mixtures have the same composition throughout the system, and heterogeneous mixtures have the oppositeDispersed phase and continuous phase The insoluble particles of a colloid do not settle down completely as the particles are small in size, usually ranging between 107 to 103 cm




Differences Between Homogeneous And Heterogeneous Ef Processes Download Table



Are All Homogeneous Mixtures Also Solutions Example
Homogeneous solution of a differential equation is the algebraic solution stemming from the solution of each derivative of the said differential equation The Inhomogeneous solution very much depends on the nature of the homogeneous solution TheA homogeneous equilibrium is one in which all of the reactants and products are present in a single solution (by definition, a homogeneous mixture ) Reactions between solutes in liquid solutions belong to one type of homogeneous equilibria The chemical species involved can be molecules, ions, or a mixture of both For the homogenous reactionHeterogeneous and homogeneous homogeneous mixture A mixture that is uniform in composition Solutions Special name given to homogeneous mixtures by chemists Phase Any part of a system that has uniform composition and properties Difference between heterogeneous and solution Heterogeneous mixtures have two or more phases and solution




Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture




The Density Bottle Strikes Again Chemical Education Xchange
The key difference between homogeneous and heterogeneous equilibrium is that in homogeneous equilibrium, the reactants and products are in the same phase of matter whereas, in heterogeneous equilibrium, the reactants and products are in different phases Equilibrium is a state in which the concentrations of reactants and products remain constantWhat is the difference between heterogeneous and homogeneous mixture Define the mixture Define homogeneous mixture Date examples of homogeneous mixtures How do you like coffee?5 rows The difference between homogeneous and heterogeneous mixture are tabulated below




Shutterstock Puzzlepix



Types Of Catalysis
Homogeneous solutionA solution composed of matter that all exists in the same state The equilibrium constants for reactions that contain substances that are all in the same phase, and reactions that contain substances in different phases, need to be calculated differently The former are called homogenous reactions, and the later are called heterogeneous reactions What is the difference between homogeneous and heterogeneous solution? Difference between homogeneous and heterogeneous mixture closed Ask Question Asked 1 year, So my question is how can we truly say if a mixture is homogeneous or heterogeneous when probability and approximations must be taken into account Browse other questions tagged aqueoussolution solutions mixtures or ask your own question




Differences Between Homogeneous And Heterogeneous Ef Processes Download Table




Differentiate Between Homogeneous And Heterogeneous Mixtures With Examples Youtube
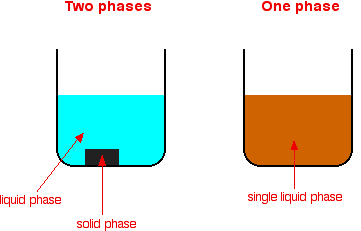
The prefixes hetero indicate difference A homogeneous mixture has the same uniform appearance and composition throughout A heterogeneous mixture consists of visibly different substances or phases The three phases or states of matter are gas, liquid, and solid What is a homogeneous and A mixture can be homogeneous having a uniform composition or heterogeneous having a nonuniform composition A homogeneous mixture is a mixture in which all substances are of the same nature and consists of similar parts Heterogeneous mixtures are made up of two or more totally different substances Homogeneous Mix vs Heterogeneous MixBy definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phase
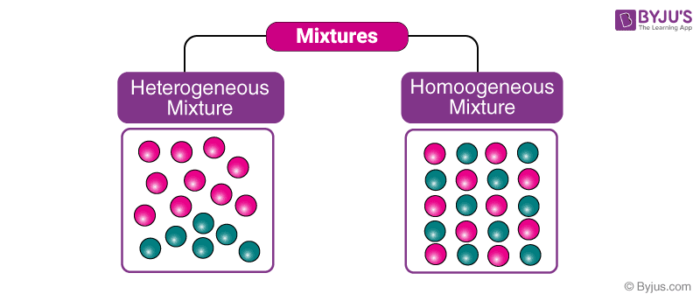



Heterogeneous And Homogeneous Mixture Differences Videos Examples




Classification Of Matter Chemistrygod




What Is Difference Between Heterogeneous And Homogeneous Brainly In




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Types Of Mixtures In Terms Of Homogeneity Science Online




How To Identify Heterogeneous Homogeneous Mixtures




Solutions And Mixtures Flashcards Quizlet



Homogeneous Vs Heterogeneous Mixture




Mixtures And Solutions Cpd Rsc Education
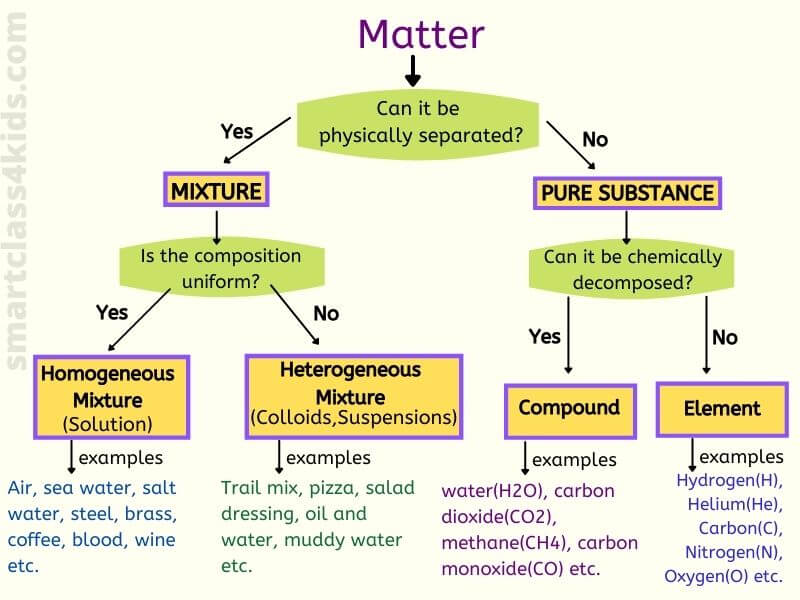



What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples




Solutions Types Of Mixtures Think You Have A Solution To This Problem Ppt Download



Q Tbn And9gctxtzdpl9qyaaesytmccdhjgig7kctubdjyeec6sj2h Ew87kwi Usqp Cau




Homogeneous Mixture Examples In Kitchen




Homogeneous Solution Of Water And Salt And Heterogeneous Mixture Of Water And Sand In Glass Beakers Stock Vector Adobe Stock




Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube




13 1 Types Of Mixtures Diagram Quizlet



Mixture




Question Video Identifying The Best Description Of A Solution From A Set Of Descriptions Nagwa
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples



Q Tbn And9gcsbe Ybqypf3mkoozl7w6krqjqo5iirs Nvzkdphtkiwz1wjxbm Usqp Cau




Heterogeneous And Homogeneous Mixture Differences Videos Examples
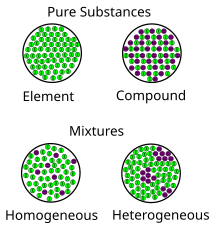



Mixture Wikipedia




Difference Between Homogenous And Heterogenous Mixture
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures




Homogeneous Mixture Examples In Daily Life




List The Points Of Differences Between Homogeneous And Heterogeneous Mixtures



Chem4kids Com Matter Solutions



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




What Is The Difference Between Homogeneous And Heterogeneous Equilibrium Slide Share
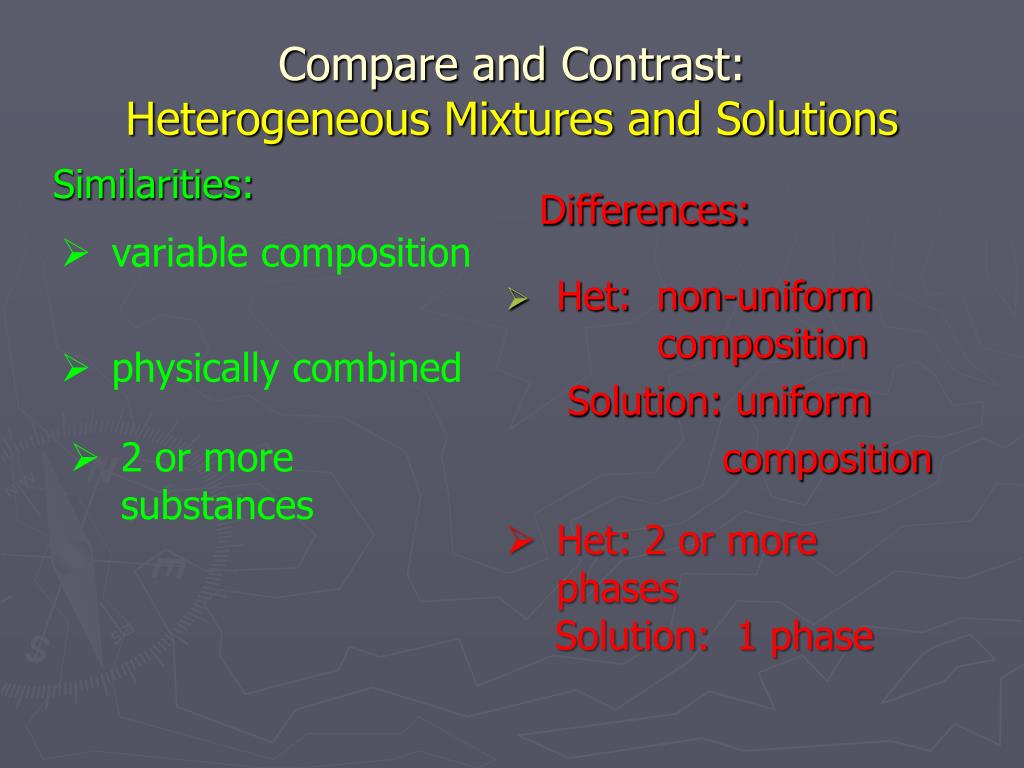



Ppt Compare And Contrast Heterogeneous Mixtures And Solutions Powerpoint Presentation Id




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous



Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous




Difference Between Homogeneous And Heterogeneous Mixtures Homogeneous Vs Heterogeneous Youtube




3 4 Classifying Matter According To Its Composition Chemistry Libretexts
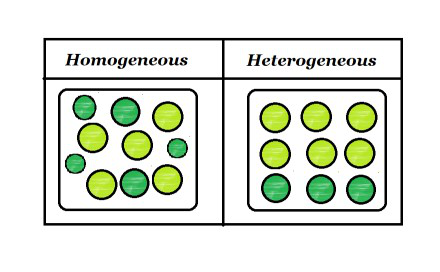



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Difference Between Heterozygous And Homozygous Scholr




What Is The Difference Between A Homogeneous Mixture And A Heterogeneous Mixture Bitwise Academy




Heterogeneous And Homogeneous Mixtures In Cooking And Learning Communities By Natalie King And Brandon Connelly Re Writing Chemistry




Examples Of Heterogeneous Mixtures Types Made Simple



Mixture




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




How To Identify Heterogeneous Homogeneous Mixtures




An Overview Of Homogeneous And Heterogeneous Photocatalysis Applications For The Removal Of Pharmaceutical Compounds From Real Or Synthetic Hospital Wastewaters Under Lab Or Pilot Scale Sciencedirect




10 Examples Of Mixtures



Homogeneous And Heterogeneous Mixture Nine Science



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora
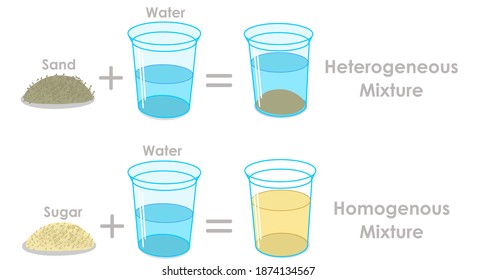



Mixtures Images Stock Photos Vectors Shutterstock




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




Mixturesandsolutions




Distinguish Between Homogeneous And Heterogenous Science Is Matter Around Us Pure Meritnation Com



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
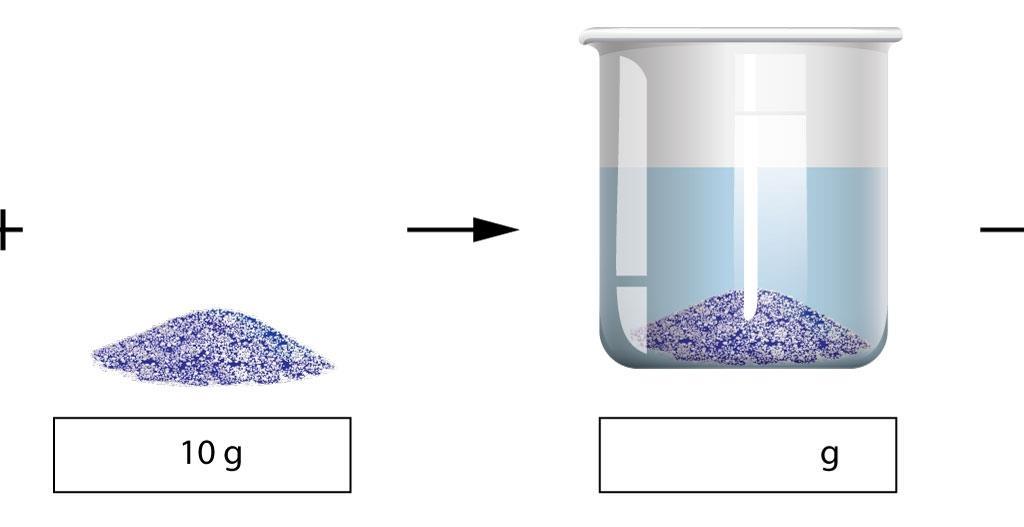



Mixtures And Solutions Cpd Rsc Education




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo




Chapter 7 Solutions I Can Distinguish Between Homogeneous And Heterogeneous Mixtures I Can Compare The Properties Of Colloids And Solutions I Can Give Ppt Download




Homogeneous Solution Of Water And Salt And Heterogeneous Mixture Of Water And Sand In Glass Beakers Chemist Chemistry For Kids Heterogeneous Mixture Chemistry
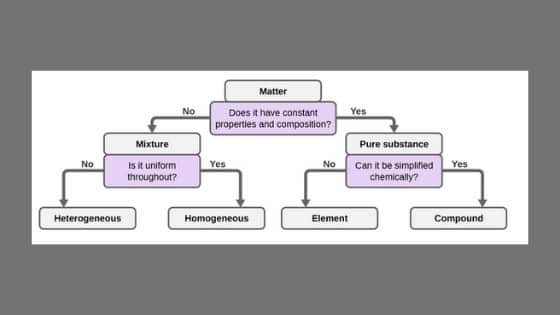



Homogeneous Mixture And Heterogeneous Mixture Ncert Books



Is There Any Difference Between Homogeneous Mixture And Solution Here On Quora Previous Answers Are Vague About This While All My Textbooks And Google Sites Say They Are Exactly Same Quora




Difference Between Homogeneous Mixture And Heterogeneous Mixture




Heterogeneous And Homogeneous Mixture Differences Videos Examples



Difference Between Homogenous And Heterogeneous Mixture Javatpoint



1




Explain The Difference Between A Homogeneous And Heterogeneous Catalyst Give An Example Of Each




Solved What Is The Difference Between Homogeneous And Heterogeneous Matter Classify Each Of The Following As Homogeneo




Ncert Solutions Science Grade 9 Chapter 2 Is Matter Around Us Pure



A Description Of Matter




Homogenous Definition And Examples Biology Online Dictionary



1




A State The Main Points Of Difference Between Homogenous And Hetergeneous Mixtures Youtube




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms




Homogeneous Vs Heterogeneous Mixtures Difference And Comparison Diffen




Interesting Chemistry Difference Between Homogeneous And Heterogeneous Mixture Chemical Chemistrynotes Chemistry Facebook




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com




List The Point Of Difference Between Homogeneous And Heterogeneous Mixture Science Is Matter Around Us Pure Meritnation Com



Heterogeneous Vs Homogeneous Solutions Chapter 12 Solutions



Classification Of Matter Webquest Handout Docx



Homogeneous Mixture Examples Chemistry




Compound Vs Mixture Difference And Comparison Diffen


